Proven reduction of cortisol in the supportive SONICS study1
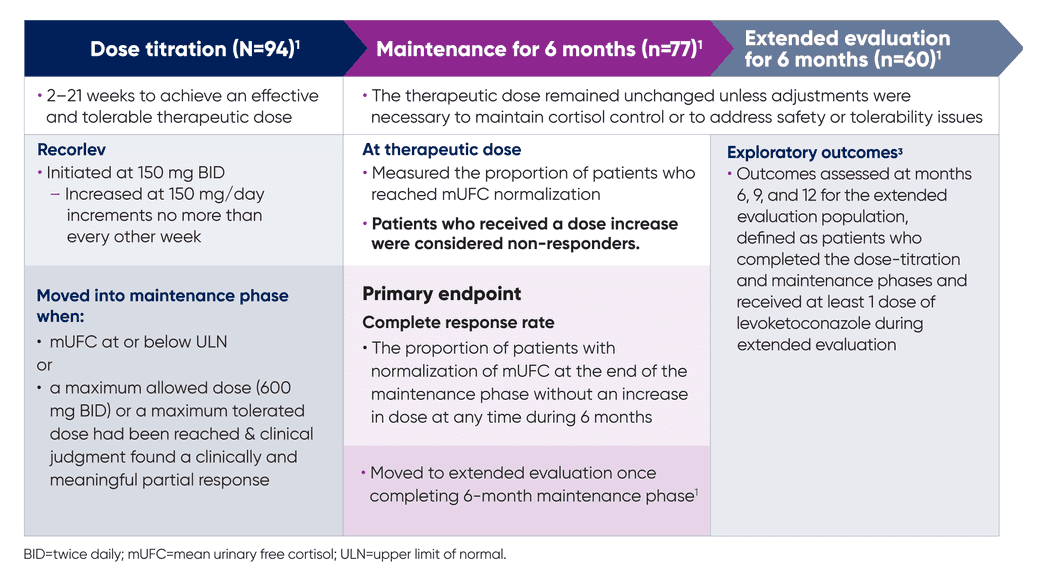
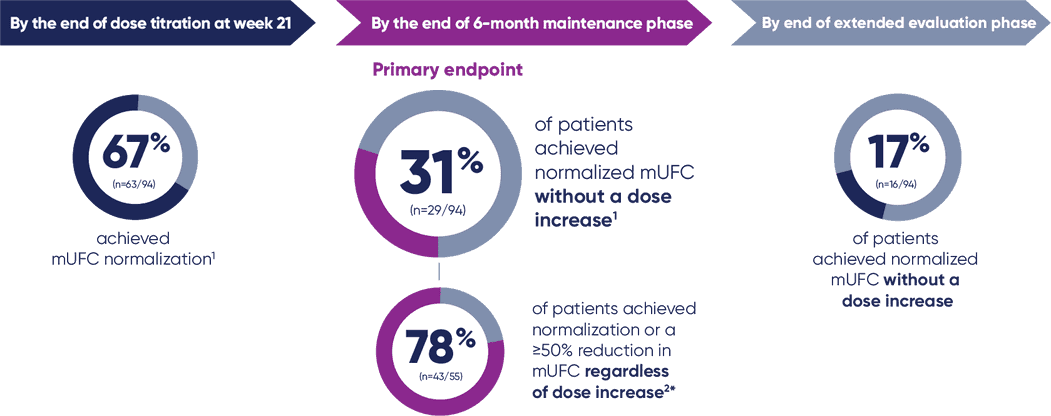
Primary endpoint
Complete response rate: The proportion of patients with normalization of mUFC at the end of the maintenance phase without an increase in dose at any time during 6 months
The SONICS study evaluated the efficacy and safety of Recorlev® in patients with Cushing’s syndrome in a multicenter, open-label study consisting of 3 phases.
Key limitations of the SONICS study are the open-label design and absence of a control group.
Complete response rate: The proportion of patients with normalization of mUFC at the end of the maintenance phase without an increase in dose at any time during 6 months
because 51% of the patients discontinued treatment prematurely due to adverse reaction, lack of efficacy, or other reasons, these results should be interpreted with caution.
BID=twice daily; mUFC=mean urinary free cortisol; ULN=upper limit of normal.
References: 1. Recorlev [prescribing information]. Chicago, IL: Xeris Pharmaceuticals, Inc 2. Fleseriu M, Pivonello R, Elenkova A, et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial [published correction appears in Lancet Diabetes Endocrinol. 2019;7(11):e22]. Lancet Diabetes Endocrinol. 2019;7(11):855-865. 3. Fleseriu M, Auchus RJ, Greenman Y. Levoketoconazole treatment in endogenous Cushing’s syndrome: extended evaluation of clinical, biochemical, and radiologic outcomes. Eur J Endocrinol. 2022;187(6):859-871.