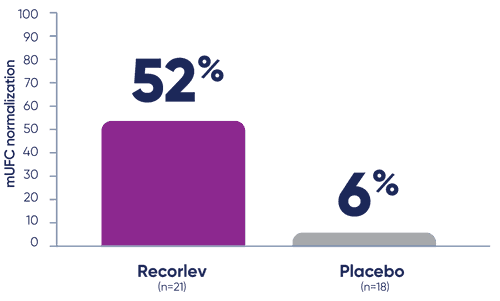
LOGICS evaluated the proportion of patients with mUFC normalization1
LOGICS had an open-label dose titration and maintenance phase for up to 19 weeks, followed by an 8-week double-blind, placebo controlled, randomized withdrawal phase and 8 weeks
of restoration.1,2
Key efficacy endpoint1
The proportion of patients with mUFC normalization, defined as a patient with mUFC at or below the ULN at the end of randomized withdrawal phase without meeting a requirement for early rescue during the randomized withdrawal phase.
- *mUFC normalization was defined as patients with mUFC at or below the ULN at the end of the withdrawal phase without requiring early rescue during the randomized withdrawal phase.2
- †Subjects eligible for the randomized-withdrawal phase achieved stable therapeutic dose (defined as the dose providing mUFC at or below the ULN, determined from 3 adequate 24-h urine collections) and maintained the dose associated with mUFC normalization for at least the final 4 weeks of the titration maintenance phase.2
At the end of the randomized withdrawal phase, significantly more patients taking Recorlev achieved mUFC normalization‡ vs placebo1
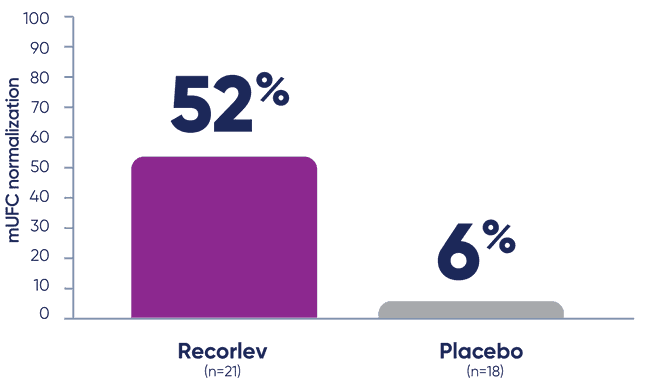
- ‡Among the 39 patients who entered the randomized withdrawal phase and had normal mUFC.
BID=twice daily; mUFC=mean urinary free cortisol; ULN=upper limit of normal.
References: 1. Recorlev [prescribing information]. Chicago, IL: Xeris Pharmaceuticals, Inc. 2. Pivonello R, Zacharieva S, Elenkova A, et al. Levoketoconazole in the treatment of patients with endogenous Cushing’s syndrome: a double-blind, placebo-controlled, randomized withdrawal study (LOGICS). Pituitary. 2022;25(6):911-926. doi:10.1007/s11102-022-01263-7